- General Top
- SEMICONDUCTOR
- STORAGE
- COMPANY
-
My ToshibaSemicon
- Semiconductor Top
-
ApplicationsAutomotive
Body Electronics
xEV
In-Vehicle Infotainment
Advanced Driver-Assistance Systems (ADAS)
Chassis
IndustrialInfrastructure
BEMS/HEMS
Factory Automation
Commercial Equipment
Consumer/PersonalIoT Equipment
Healthcare
Wearable Device
Mobile
Computer Peripherals
-
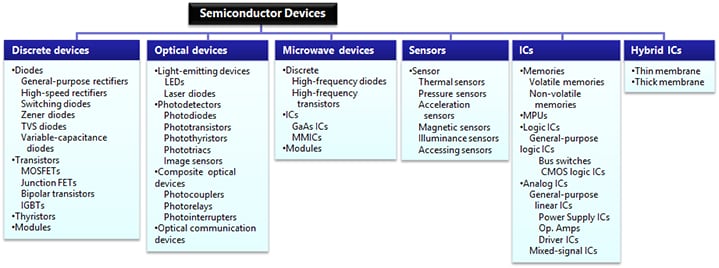
ProductsAutomotive Devices
Discrete Semiconductor
Diodes
Transistors
Logic ICs
Analog Devices
Digital Devices
Wireless Devices
※
: Products list (parametric search)
Power SemiconductorsSiC Power Devices
※
: Products list (parametric search)
Isolators/Solid State RelaysPhotocouplers
Digital Isolators
Solid State Relays
Fiber Optic Transmitting Modules
※
: Products list (parametric search)
MOSFETsIGBTs/IEGTsBipolar Transistors※
: Products list (parametric search)
Diodes※
: Products list (parametric search)
MicrocontrollersMotor Driver ICsIntelligent Power ICs※
: Products list (parametric search)
Power Management ICsLinear ICs※
: Products list (parametric search)
General Purpose Logic ICsLinear Image SensorsOther Product ICsOther Product ICs
※
: Products list (parametric search)
-
Design & Development
-
Knowledge
- Where To Buy
- Part Number & Keyword Search
- Cross Reference Search
- Parametric Search
- Stock Check & Purchase
This webpage doesn't work with Internet Explorer. Please use the latest version of Google Chrome, Microsoft Edge, Mozilla Firefox or Safari.
require 3 characters or more. Search for multiple part numbers fromhere.
The information presented in this cross reference is based on TOSHIBA's selection criteria and should be treated as a suggestion only. Please carefully review the latest versions of all relevant information on the TOSHIBA products, including without limitation data sheets and validate all operating parameters of the TOSHIBA products to ensure that the suggested TOSHIBA products are truly compatible with your design and application.Please note that this cross reference is based on TOSHIBA's estimate of compatibility with other manufacturers' products, based on other manufacturers' published data, at the time the data was collected.TOSHIBA is not responsible for any incorrect or incomplete information. Information is subject to change at any time without notice.
require 3 characters or more.
Semiconductor raw materials
Download "Chapter I : Basis of Semiconductors" (PDF:1.2MB)
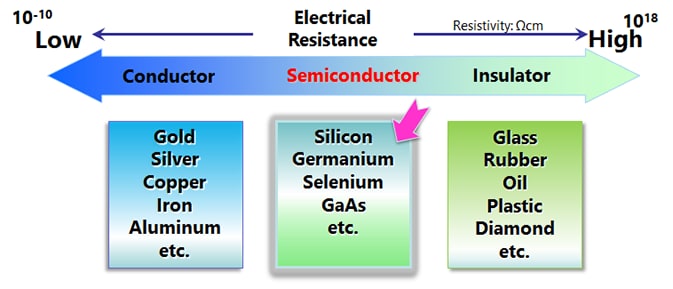
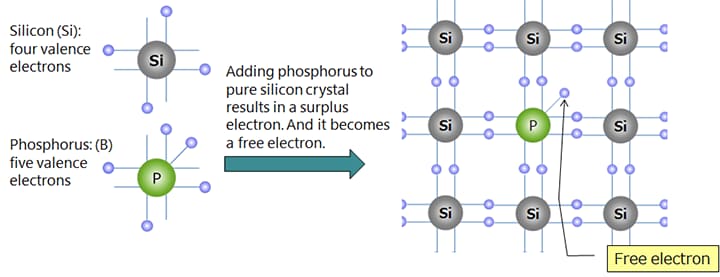
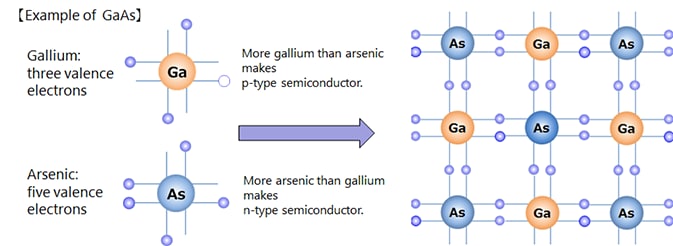
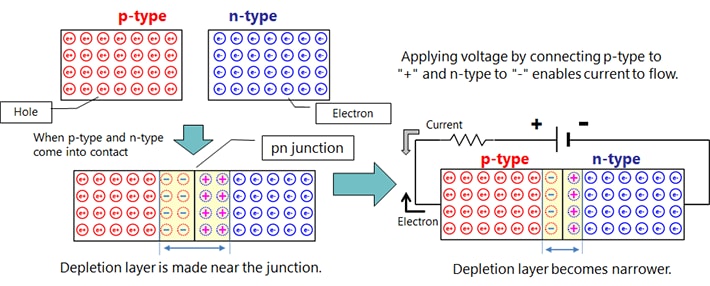
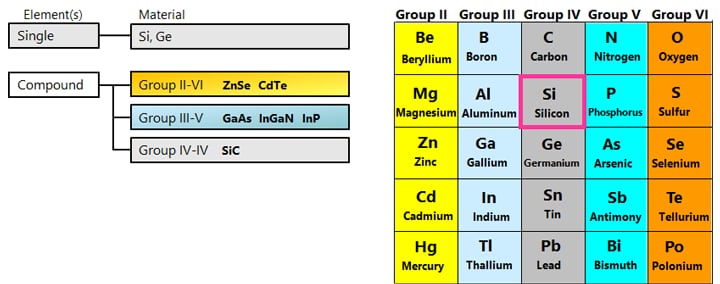
Silicon (Si) and germanium (Ge), which are the main raw materials for semiconductors, belong to Group IV.* Pure crystals (intrinsic semiconductors) have properties close to those of an insulator. However, adding trace amounts of impurities (diffusion or doping) significantly reduces the electrical resistance and makes them exhibit the properties of a conductor. Depending on the type of impurity added, n-type and p-type semiconductors can be formed. Impurities used to form n-type semiconductors include phosphorus (P), arsenic (As), and antimony (Sb). Impurities used to form p-type semiconductors include boron (B), gallium (Ga), and indium (In).
Compound semiconductors are made from multiple elements, unlike silicon semiconductors, which are made from a single element. Examples of combinations include elements from group III and group V, group II and group VI, and group IV elements. SiC and GaN, which are wide band gap semiconductors that have been in the spotlight recently, are also compound semiconductors.
*: Currently, the long periodic table is generally used, but the short periodic table is used here because it is easier to explain the properties and behavior of the elements that are the raw materials for semiconductors, as they are arranged based on the electron shells of the atoms. In the short periodic table, the groups of elements are designated by Roman numerals, while in the long periodic table, they are designated by Arabic numerals, and their values are also different. For example, Si is both in group IV and group 14.
Chapter I : Basis of Semiconductors
Related information
- Products
- FAQ